Combat Yeast Stress via iStill's Burst Protocol!
Introduction
Fermentation has long been frowned upon, among craft distillers. Most believed that fermentation was simply the place where your base alcohol was made. The followers of the iStill Blog know that I have made it my personal crusade to inform the industry that fermentation is not just about alcohol production, but also about flavor production. The better you control your fermentation, the better flavors you can distill into your spirits.
Today we take another step down the fermentation rabbit hole. Let's see how deep it really is. Let's dive in deeper and establish another level of fermentation knowledge, for even more control and even better flavors! Because that's where you need to shine, dear craft distiller: at the production of better tasting spirits, in the understanding that better taste molecules are mostly formed during fermentation.
Fermentation science
When yeast is introduced to a mash, a liquid with fermentable sugars present, the yeast will first - as long as there's oxygen - grow and reproduce. As oxygen levels in the mash drop, the yeast cells stop propagating and turn on their secondary metabolism: the one that turns those fermentable sugars into alcohol and where CO2 is produced as a by-product.
When fermenting in a (relatively) sealed vessel, at atmospheric conditions, like - well - in an ordinary fermenter with water-lock, CO2 is produced and partially released. This CO2 that gets released from the fermentation quickly replaces any headspace and any remaining air or oxygen above the ferment. Why? Because part of the CO2 that's released bubbles out of the fermenting liquids. And because CO2 is heavier than air. There you have it: part of the CO2 bubbles up and is released, while another part of CO2 remains trapped inside the fermenting liquids.
The concentration levels of dissolved CO2 within the fermenting liquid are based on sugar levels, alcohol percentage, and temperature. In general, the amount of CO2 will range from ∼1.4 to 2 g/L, when brewing between 12 °C and 25 °C. Higher temperature fermentations are often the result of faster fermentations, and as fermentation is an exothermic chemical process, that creates a lot of heat, faster ferments associate with (and cause!) more CO2 production and warmer temperatures.
Chen and Gutmanis (1976) reported significant inhibition of yeast growth above ∼0.65 g/L CO2 dissolved in the media. Given the above, this makes perfect sense. As oxygen is depleted, yeast switches to its secondary metabolism, that does no longer support yeast cell growth and propagation. Instead, in this secondary survival mode, sugars are turned into alcohols, and CO2 is produced as the aforementioned by-product.
Furthermore, these researchers reported a 20% reduction in fermentation activity when CO2 concentration dissolved in the fermenting liquids reached ∼1.1 g/L. High concentrations of CO2, dissolved in the fermenting liquids, were shown to affect yeast viability and induce a general stress response. Concentrations of dissolved CO2 in the ferment were shown to impact yeast viability.
Summary
A summary? As yeast is added to the mash, it consumes oxygen and reproduces. As oxygen levels deplete, yeast switches its metabolism, where it stops reproducing, and starts producing alcohol and CO2. The level of about 0.65 g/L of dissolved CO2 seems to be the threshold where propagation stops and where alcohol production starts. All is good so far, but ... as dissolved CO2 levels rise, they start to harm the yeast's viability to produce alcohol (ethanol) as well as flavors (ester molecules). Too much CO2 harms the alcohol and flavor production of your fermentation! A level of 0.65 g/L is good, a level of 1.1 g/L is bad already to the extent that it deteriorates the yeast performance, both in terms of alcohol and flavor production, by 20%. Higher levels of dissolved CO2 will further impact yeast performance negatively.
More scientific proof of CO2-induced yeast stress
Landaud et al. (2001) studied the effect of different CO2 concentrations on the free amino nitrogen (FAN) consumption rate of yeast during fermentation. These researchers found that the FAN consumption rate of yeast was 300% faster when the CO2 concentration was reduced from 3.65 to 1.78 g/L. In laymen's terms? Higher CO2 levels hamper the yeast's ability to consume "food" and get access to the energy they need to get the job (of alcohol and ester production) done.
Knatchull and Slaughter (1987) reported a reduction in the absorption of branched-chain amino acids by yeast cells, resulting in a reduction of higher alcohols and esters when fermenting under high CO2 concentrations. What this means in plain English? That they found out, via scientific research, that higher levels of dissolved CO2 result in less tasty fermentations.
Problem definition
High levels of dissolved CO2 stress-out the yeast. As a result, the yeast produces lower amounts of both alcohols and esters. The consequence is that you, as a craft distiller, have a less efficient and a less effective fermentation cycle, resulting in lower and slower yield, and in less flavorsome spirits.
That is a problem. That is a BIG problem. It is a big problem, because the craft distiller's business case depends on flavor and yield. No, the craft distiller is never going to out-produce Big Alcohol on costs per liter or production efficiency. And as this automatically results in higher production costs for craft distillers, two existential questions emerge from that conclusion:
- How can the craft distiller produce as efficiently as possible, to obtain the lowest possible production costs?
- Can craft distillers create better tasting spirits that warrant the higher price per bottle, relative to Big Alcohol?
An efficient fermentation that generates the best flavors possible is KEY to your operation. It is essential to the long term viability of the craft distilling industry. It is what gives us, craft distillers, our place in the market.
High levels of dissolved CO2 hamper the craft distiller's alcohol production efficiency and flavor production effectivity, and are - therefore - a direct and ominous threat to your value proposition specifically, and the long-term success of the craft distilling industry in general.
Solution
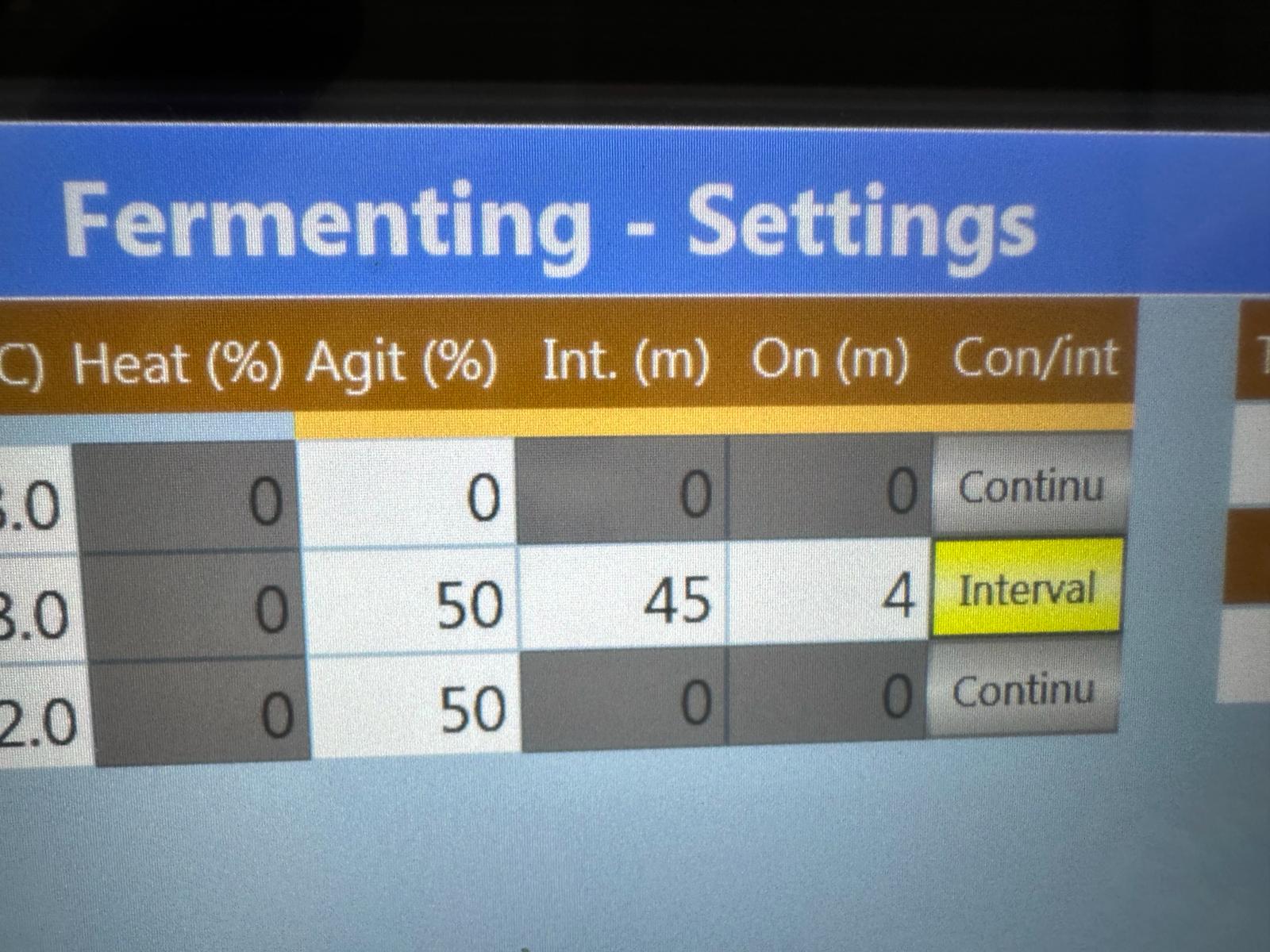
iStill has invented a way for you to control the levels of dissolved CO2 during fermentation. We call it the iStill Burst Protocol. It is a piece of control, a new software patch basically, that we added to our fermenters and the iStills (that can also be used as fermenters), some time ago.
What the iStill Burst Protocol does, is that it allows you to choose short bursts of agitation at specific intervals. What the burst agitation protocol does, is that it pushes big amounts of dissolved CO2 out of solution. If you set a specific interval, this means that you release dissolved CO2 on a regular basis, constantly making sure that the yeast, your yeast, operates within its best performance window.
An example
Imagine that you want to ferment a molasse batch into a rum wine. Molasses ferments quickly, so you'll see a lot of CO2 build-up and a warm if not hot ferment. Molasses are high in ashes and in unfermentable sugars, conditions that already induce yeast stress. High temperatures and high levels of dissolved CO2 add more stress to the yeast, potentially deteriorating your results, leading to lower yield and off-flavors in your rum wine and - later - in your rum.
Here's an example how the iStill Burst Protocol helps:
- Choose a 36 hours fermentation time;
- Add the yeast and do not disturb the fermentation for the first 8 hours;
- So that yeast growth and propagation are not hampered;
- After 8 hours, when oxygen levels are depleted and CO2 is on the rise ...
- Do 4 minutes iStill Burst Agitation periods every 45 minutes from then onwards;
- Now, during the alcohol and ester production phase ...
- The levels of dissolved CO2 are reduced every 45 minutes ...
- Which leads to healthier and les stressed-out yeast ...
- Resulting in higher yield and better flavors, via a faster production of alcohols and esters!
iStill's Burst Agitation Protocol: an amazing USP!
Our research shows that iStill's Burst Agitation Protocol helps produce 20% more alcohols and flavors in only 60 to 80% of your normal throughput time! How's that for lowering your production costs and improving the amount of flavor you sell per bottle?
Do you want to learn more about this amazing new innovation? Please reach out to Veronika@iStillmail.com and register for the hands-on Master Distillers Course. We still have a few spots available in April.
Do you want to order an iStill distillery or an iStill fermenter? Email me directly at Odin@iStillmail.com, so that we can plan a video call for us to discuss your plans and our amazing distilling solutions.
Choose the iStill Burst Protocol ...
www.iStill.com
